Artificial Intelligence for Health Imaging (AI4HI) is a network of 5 large EU-funded research projects (Chaimeleon, EuCanImage, INCISIVE, ProCancer-I, PRIMAGE), consisting of more than 120 institutions from 20 countries. This network is currently collecting images from more than 91,000 patients with different types of cancer and is working on artificial intelligence (AI) solutions that will be trained and validated on the collected medical images and related clinical and molecular data to improve clinical practice through AI-enabled clinical decision support solutions. A new EU co-funded effort, CancerImageEurope, has very recently been added to the above AI4HI projects striving to integrate and federate even large volumes of data. This new project, starting in January 2023, is a joint effort by the AI4HI Network and major European Research Infrastructures (Euro-BioImaging, BBMRI, EATRIS and ELIXIR) and aims at building up a hybrid (distributed and centralized) infrastructure integrating all major existing European Real World Data infrastructures on cancer images, including all types of cancer.
To ensure clinical adoption and impact on oncologic imaging, we believe that the AI-based services that are under development need to have a clearly defined medical focus and must be validated through carefully designed clinical validations; they also need to be trustworthy, i.e. technically robust, clinically safe, as well as ethically and legally compliant. Hence, we developed international consensus guidelines for designing, developing and deploying trustworthy AI tools: Fair, Universal, Traceable, Usable, Robust and Explainable (FUTURE-AI guidelines). RadioVal is a new EU-funded that will build and validate trustworthy AI solutions for patient selection of neoadjuvant chemotherapy in breast cancer by leveraging the large-scale datasets collected in the AI4HI projects and based on the FUTURE-AI guidelines.
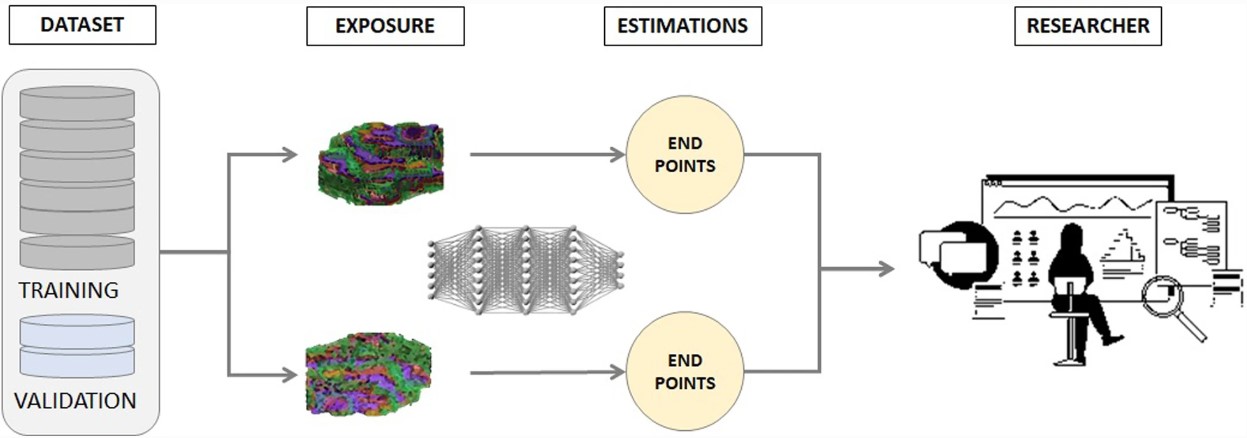
Furthermore, research on large volumes of multi-center, heterogeneous datasets allow AI-supported observational, analytical, and in silico predictive studies and decision-support services to be constructed in oncology. The availability of cancer imaging and other relevant clinical big data allows researchers to obtain and document, post hoc, tumor phenomena and associate them with different outcomes based on real-world data (RWD), also considering the relevant temporal dimension (e.g. tumor radiomics for the estimation of overall cancer-free survival). The development and training process of the AI models does not entail any risk for the cancer patient as the researcher does not have any active intervention in the clinical course of the individuals being studied, given that the exposure and endpoints have already occurred before the start of the RWD collection.
To ensure clinical use, the characteristics of the subjects who will take part in the study, including context, exposures, timing, confounders, and interactions, have to be well-defined before data collection from existing health records. Furthermore, clinical end-points must be carefully and precisely defined, whether these relate to diagnosis, treatment, prognosis or follow-up. Examples of variables to be used as inputs to the AI models include demographic, clinical-analytical, pathology, imaging, various annotations, and treatment. AI models are developed, trained, tested, and validated in silico on datasets from large repositories, such as the AI4HI repositories, linking the multicenter extracted radiomic information with the relevant molecular and clinical data. Within repositories, data homogeneity at the semantic and technical level is an important pre-requisite, and a step that all AI4HI projects are taking before making the data available for AI training. Equally important is that collected aggregated multilevel data (clinical, molecular, imaging) is properly deidentified to meet the General Data Protection Regulations (GDPR) requirements. As research is performed on retrospectively collected RWD, patient informed consent is usually waived by the Ethics Committee at the data provider sites. Patient consent is required if data is prospectively collected before the episode is finalized. All these aspects are important for clinical translational when executing studies involving AI, RWD extraction, and validation.
Key points
- EU-funded research projects address the creation of AI-supported clinical decision support solutions.
- AI-based models in oncologic imaging need to be fair, robust, and trustworthy.
- Appropriate definition of relevant study parameters is essential to ensure clinical adoption.
- Clinical validation phases of AI-based clinical decision support systems need careful design.
Authors: Luis Marti-Bonmati, Dow-Mu Koh, Katrine Riklund, Maciej Bobowicz, Yiannis Roussakis, Joan C. Vilanova, Jurgen J. Fütterer, Jordi Rimola, Pedro Mallol, Gloria Ribas, Ana Miguel, Manolis Tsiknakis, Karim Lekadir & Gianna Tsakou