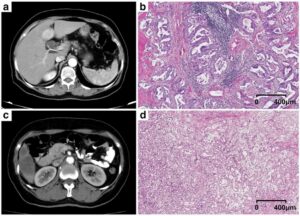
A myriad of tools is being created that use AI for medical imaging, but the community needs to be sure that the technology also works in clinical practice. This is only possible if AI is introduced in a responsible way, according to Wiro Niessen, a professor in Biomedical Image Analysis at Erasmus MC, Rotterdam and Delft University of Technology, and director of the Biomedical Imaging Group Rotterdam.
“Radiologists, rather than asking themselves if they’re going to lose their jobs, should wonder how they are going to use AI to its full potential,” he said in an exclusive interview during the last European Congress of Radiology. Machine learning developers, the academia and the industry must all start collaborating in order to design strategies for a successful introduction in clinical practice.
Future relevant clinical applications
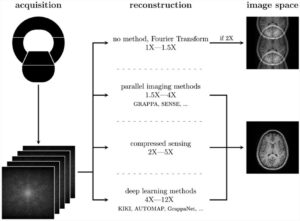
AI will have a profound impact from image acquisition to therapy and therapy-monitoring outcome, for all disease areas, from cardiovascular to neurology, oncology, MSK and many more. Particularly relevant will be its role in image information and analysis. A lot of input data from sensors are available in an image, which can be extracted and mined. This can be done with CT and MR reconstruction algorithms, but also using a neural network, to learn the relationship between the raw data of the scanner and the image. This may allow faster screening, further dose reduction and better image quality, Niessen explained.
“This is an exciting area. In the coming years, we will increasingly see AI solutions that are already integrated at the moment the image is being taken,” he said. AI will help better organise radiology departments and improve their efficiency, especially when resources are scarce. AI will help ease workflows in a number of occasions; for instance, by helping staff identify the most urgent cases.
Smooth integration into workflow
Deploying an algorithm should be easy so that the workflow isn’t altered too much. “We have to make sure that these algorithms are seamlessly integrated so that when you open a case, all the information is already there and you don’t have to launch a different application every time you need information. Ideally, you need to use a solution that has done all the image analysis beforehand, underwater,” he said.
Researchers are trying to improve the user experience, but it is still going to take a while before they develop a perfect product. “We’re in the early days and the first solutions are not going to be perfect, so it’s going to take a lot of user feedback and user experience perspective,” Niessen said.
Responsible innovation
Innovators will need to show that imaging and imaging combined with AI has additional value for the patient. “With evidence-based healthcare, we need to show that we are currently underusing all the data contained in imaging studies and that AI will help to harness that data. There is a lot of information in this imaging data that will improve treatment, and the consequences of missing a case are very costly,” he said. Exciting tools are emerging not just from the market, but also academia, and from its cooperation with the industry. Niessen, who is Chief Scientific Officer of Quantib, Erasmus MC’s spin-off company, said they were currently working to increase the scale of their experiments. “Good data is essential if you want to do data science. You want the data on which you train your algorithm to be as representative as possible for a large number of things you see in clinical practice. You have to mine data from different centres, so we’re increasingly building partnerships with training hospitals,” he said.
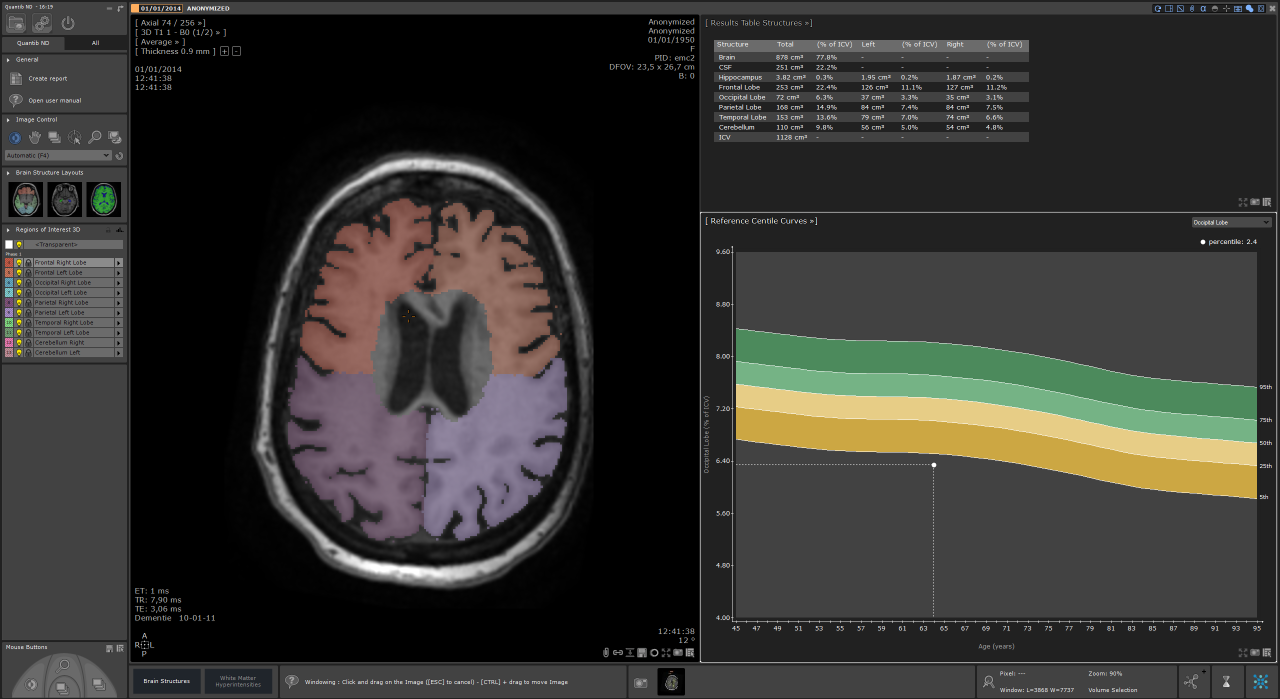
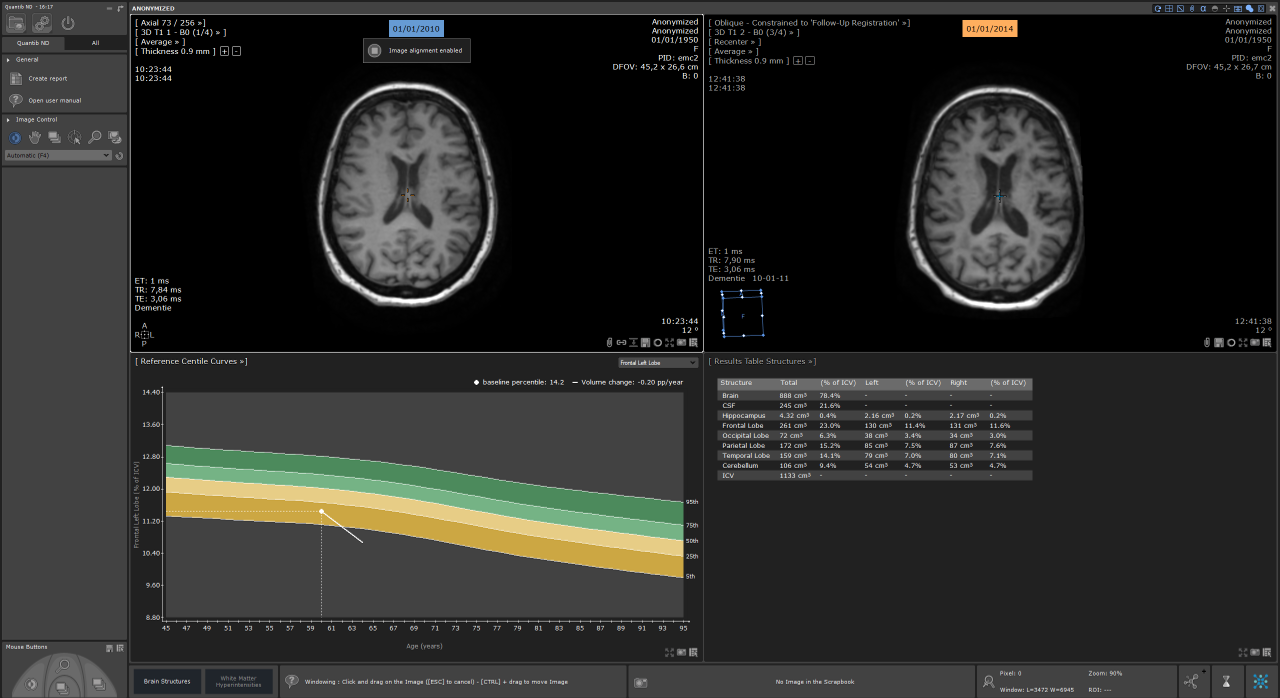
Quantib has recently received FDA clearance for two of its products: Quantib Brain, which measures changes in the brain related to Alzheimer’s disease and MS using segmentation, quantification of the volumes of grey matter, white matter and CSF, and tracking changes in white matter hyperintensities; and Quantib ND, a similar solution that also measures changes in the brain on a more granular level, by segmenting lobes, hippocampus and total brain vs. CSF. These are exciting times as the first line of products that have integrated machine learning and deep learning using data from other studies are now receiving FDA approval and a CE mark. “That’s the first step towards the dot on the horizon, in which a patient is now treated with all the knowledge from previously ill patients,” he concluded.

Wiro Niessen is a Full Professor of Biomedical Image Processing in the Departments of Radiology and Medical Informatics at the Erasmus MC. His research interests include many aspects of computer vision, biomedical image analysis, and computer-assisted interventions. He is also Professor at Delft University of Technology, at the faculty of Applied Sciences, and director of the Biomedical Imaging Group Rotterdam. Prof. Niessen is Chief Scientific Officer of Quantib, Erasmus MC’s spin-off company.